New Findings on Nickel Complexes Improve Efficiency in Chemical Applications
Researchers at the University of Chemistry and Technology in Prague have published the study of Tröger's base (TB) and spiro-Tröger's base (spiroTB) derivatives.
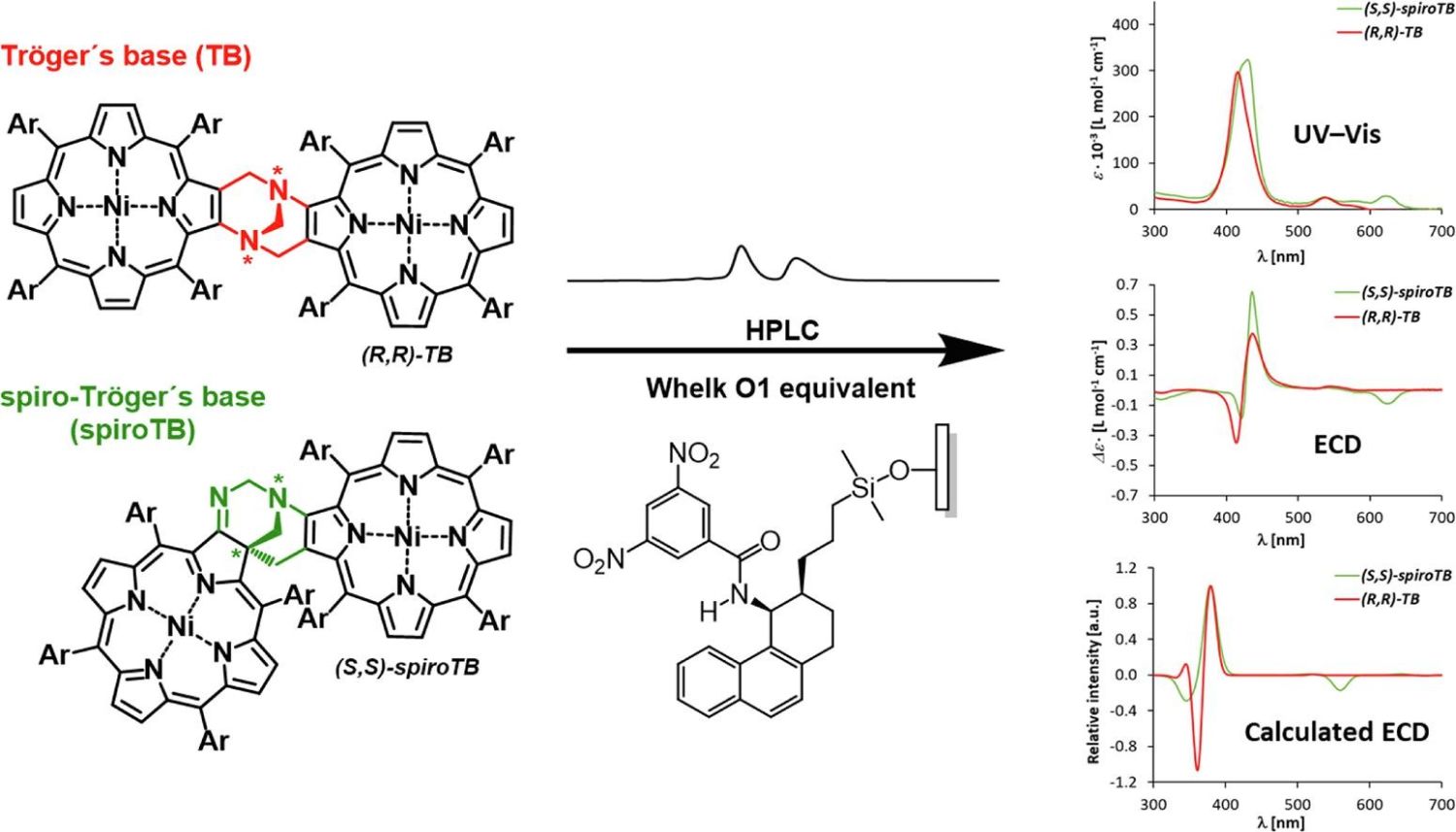
Figure 1: Graphical abstract
Utilizing high-performance liquid chromatography (HPLC) on an analytical ReproSil Chiral-NR column, the researchers successfully enantioseparated racemic mixtures of nickel(II) complexes with phenyl or 3-methoxyphenyl substitutions. They achieved high purity and determined the absolute configurations of the isolated enantiomers using electronic circular dichroism (ECD) spectra, supported by time-dependent density functional theory (TDDFT) calculations.
The research highlights several key findings. First, the chromatographic resolution of spiroTB enantiomers was superior to that of TB enantiomers, achieving high molar ellipticity values of up to 2.15 ∙ 10^7 deg ∙ cm^2 ∙ dmol^−1. Second, spectroscopic analysis combining UV-Vis and ECD spectroscopy with TDDFT calculations confirmed the absolute configurations of all compounds, showing excellent agreement between experimental and simulated spectra. Third, the study presents the first preparative enantioseparation of spiroTB derivatives, marking a significant advancement in chiral recognition research.
Bohumil Dolenský, corresponding author of the study, commented on the implications of their findings: "We believe that high molar ellipticity values and superior enantioseparation of nickel(II) porphyrin-chlorin spiroTB over TB, together with its ability to absorb light and act as a catalyst, will attract the attention of scientists in various fields."
The research underscores the potential applications of spiroTB derivatives in catalysis and chiral recognition, providing a new pathway for the development of advanced materials and biochemical systems.
Text is based on the research article:







