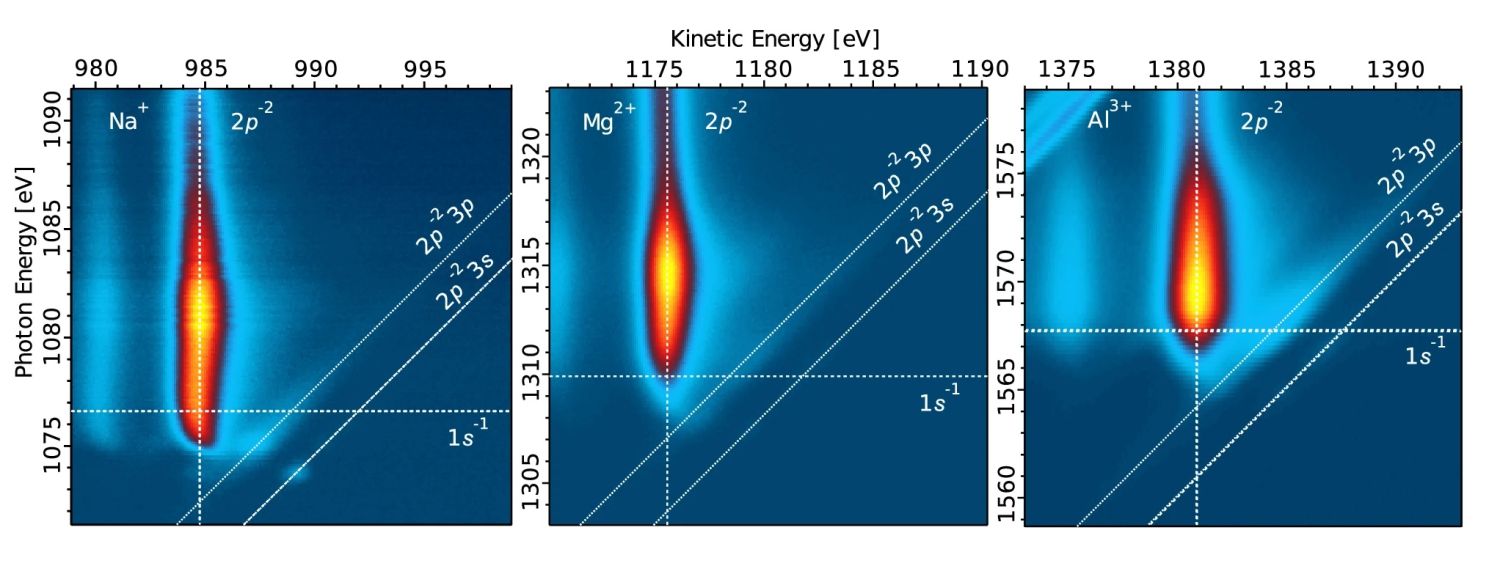
How quickly does an electron sink in water?
Prague, 16.10.2024 – Electron transfer dynamics are of interest to all chemists worldwide, because substances use electrons to interact with each other. These interactions take place very quickly, and this necessitates attosecond level experiments to describe and investigate the interactions. UCT Prague research teams led by Eva Muchová and Petr Slavíček, together with colleagues from Uppsala University in Sweden and the Fritz Haber Institute in Germany, found that ultrafast electron dynamics can be investigated using liquid-jet photoemission spectroscopy. The researchers were the first in the world to describe, a recent Nature Communications article, the X-ray-induced electron transfer that occur following excitation or ionization of the 1s electron of the hydrated Na+, Mg2+, and Al3+ ions.
„What happens to an electron after it leaves an ion in an aqueous environment is a sexy scientific topic described from a number of angles. But what was still missing in this puzzle was the time period after the birth of an electron and its separation from an ion,“ says Associate Professor Muchová.“„Everything happens so quickly that with a time-resolved experimental method, we would need subattosecond laser pulses, which is not yet possible, since we don‘t know if it would even work properly yet. So we went about our experiments differently,“ explains Associate Professor Muchová.
By the word „differently“, Muchová refers to map the event in the energy domain and not in the time domain. The experimental research teams used the core-hole-clock method to create a 2D electron signal map. From this information, the research teams were able to infer the delocalization timescales, e.g. a timescale in which the electron initially localized near the ion delocalizes over the surrounding water molecules.
The experiments themselves, conducted using the DESY synchrotron in Hamburg and analysed at UCT Prague, showed that the period of time for when an electron is „taken away by water“ from an ion depends on the strength of an ion‘s electric charge, and thus, on the way the surrounding water molecules are organized. The „carefree father who lets his child swim“ is, in this case, sodium. Aluminium is like a fierce protector, and magnesium is somewhere in between.
What are the broader implications for Associate Professor Muchová’s research team and her colleagues? Understanding another part of the complex and diverse process of electron transfer using a more accessible technology that can be used to study fundamental processes ranging from voltage transfer in biological organisms to photocatalysis, (also in the solid phase).
Reference
Nature Communications: Attosecond formation of charge-transfer-to-solvent states of aqueous ions probed using the core-hole-clock technique